Servicing, validations, and calibration laboratory
Servicing, validations, and calibration laboratory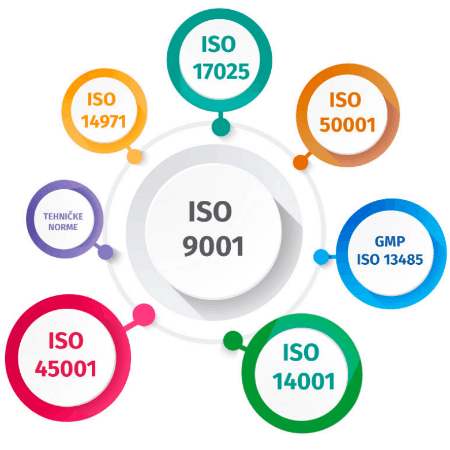

As a leading manufacturer of clean rooms, ventilation, and air conditioning equipment, Klimaoprema offers a complete turnkey service,
which, in addition to the design, production, and construction of clean rooms, includes servicing and regular preventive maintenance, as well as validation and calibration of measuring devices.
At Klimaoprema, we follow a quality system with the following integrated standards:
• Quality management system (ISO 9001);
• Environmental management system (ISO 14001);
• Occupational health and safety management system (ISO 45001);
• Medical devices management system (ISO 13485);
• Energy management system (ISO 50001);
• General requirements for the competence of testing and calibration laboratories (ISO 17025).
The requirements of the corresponding technical standards form part of the integrated management system.
which, in addition to the design, production, and construction of clean rooms, includes servicing and regular preventive maintenance, as well as validation and calibration of measuring devices.
At Klimaoprema, we follow a quality system with the following integrated standards:
• Quality management system (ISO 9001);
• Environmental management system (ISO 14001);
• Occupational health and safety management system (ISO 45001);
• Medical devices management system (ISO 13485);
• Energy management system (ISO 50001);
• General requirements for the competence of testing and calibration laboratories (ISO 17025).
The requirements of the corresponding technical standards form part of the integrated management system.
Services we provide
• HVAC balancing
• Qualification of clean rooms and devices
• Testing the integrity of the HEPA filter
• Particle counting
• Room recovery test
• Airflow visualization test/Smoke study
• Temperature mapping/monitoring
• Balancing the distribution of technological media (refrigerants, domestic hot and cold water).
• Preparation of validation, qualification, and requalification documentation pertaining to HVAC, technological media, clean rooms, temperature-controlled equipment, and other equipment in the same or similar domain (pass box, LF cabins, and cabinets, BSC Class II cabinets, refrigerated chambers, refrigerators, warehouses, temperature-controlled rooms...).
• Calibration of differential pressure, temperature, and relative humidity meters


Servicing and validation

Full or partial maintenance
- Total care of the plant, system or device
- Contractual maintenance
- Emergency interventions within 0-24 hours from reporting the failure
- Partial services
- Preventive inspections and replacement of spare parts
- Qualifications / Validations / Calibrations at the request of clients

Preparation of validation and qualification documentation
We offer preparation of the following documentation:
• URS – User requirement specification,
• GMP RA – Risk assessment,
• RDS – Room data sheet/Roombook,
• FS – Functional specification,
• TS – Technical specification,
• DQ – Design qualification,
• MC – Mechanical Completion procedure,
• COMMISSIONING – FAT/SAT,
• IQ – Installation qualification,
• OQ – Operation qualification,
• PQ – Performance qualification,
• TEMPERATURE MAPPING – Cold or Hot Rooms,
Chambers, Warehouse
• Sensor calibration certificates
• Preparation of documentation using advanced programs that ensure good documentation practice (GDP) – COMOS, DMS, and KNEAT




Professional qualifications of the validation team:

Calibration laboratory
We are currently able to calibrate the following:
• Temperature meters in the range from -35°C to +165°C
• Differential pressure meters from -5000 Pa to +5000 Pa
• Relative humidity meters in the range from 5 to 95% RH.

Professional qualifications of the calibration laboratory:
